- Dating a fossil is very important, and without dates, it is difficult to understand the context
- It is most common to date the sediments surrounding the fossil rather than directly date the fossil
- Relative dating is important for understanding the sequence of geological events that occurred in association with a fossil
- Absolute dating helps geologists calculate an exact age of a fossil
Highlights
The age of a fossil is one of the most important details to know when studying the evolutionary history of life on Earth. It is how scientists place a fossil into context and develop a greater understanding of how, and when, that organisms lived. Without the age of a fossil, we would never know how old the famous hominin, “Lucy,” was or the age of the oldest stone tools. These discoveries are essential for understanding what makes us human. But, how does a scientist know exactly how old a fossil is?
Dating methods
To properly date a fossil, geologists use two different methods: relative and absolute dating. Relative dating puts events in sequence of chronological order without determining the exact age. Absolute dating determines the numerical age of the fossil by either directly dating the fossil or indirectly by dating of material associated with the fossil. It is important to note that fossils are very rarely directly dated due to how fragile they are and how destructive some dating methods can be. The methods discussed here mainly focus on how fossils are dated by determining an age for the sediments they are discovered in.
There are a many different types of absolute dating methods and which one to use depends on the setting and type of sediment or rock the fossil is buried in. Geologists will often use multiple dating methods to ensure a proper age is calculated. This is important because if a fossil is incorrectly dated, it can skew our understanding of critical events in our evolutionary history.
The Laws of Stratigraphy
Relative dating depends on the “Laws of Stratigraphy,” which were first developed by Nicolas Steno (1638–1686). These laws, as detailed below, try to explain how layers of sedimentary rocks (i.e., strata) formed at Earth’s surface as well as any processes that affected their deposition (Figure 1 below).
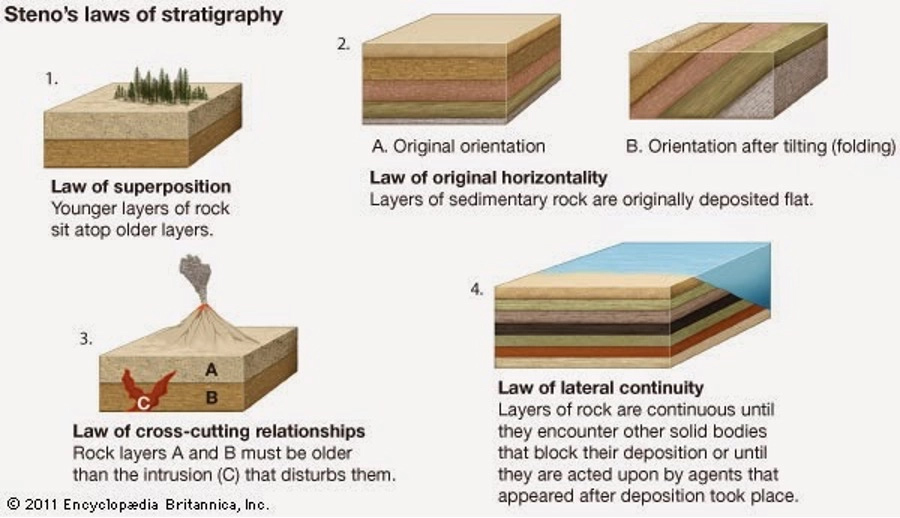
Figure 1. Nicolas Steno’s laws of stratigraphy.
Image source britannica.com
- The first principle, also referred to as the law of original horizontality, states that stratigraphic layers are deposited horizontally.
- The second principle, the law of lateral continuity, states that these layers will continue to extend horizontally until the environment changes. A great example of this can be seen at the Grand Canyon. If you look out at the layers of rock exposed in the canyon, in some areas it almost looks like you can connect the layers from one side of the canyon to the other (Figure 2 below). This is because at one time they did connect, and it was not until the Colorado River came rushing through and eroded the canyon that we see the formations of today.
- The third principle is the law of superposition, which states that the oldest layer of rock will be at the bottom of a sequence and the youngest will be at the top. This means if there were no disturbances in how the layers of sediment in the Grand Canyon accumulated—the layers at the bottom of the canyon will always be older than the layers at the top of the canyon.

Figure 2. Photo of Grand Canyon. Note the horizontal layers that appear to have connected at one point in time.
Image credit Jayde Hirniak
Relative and Absolute dating
So how does this all relate to relative dating? These laws allow scientists to put sediments/rock units and geological events in order and potentially link strata from multiple locations. If fossil A is found at the top of a sequence and fossil B is found at the bottom of the sequence, it can at least be determined that fossil A is younger than fossil B. While useful, relative dating cannot determine the exact age of a fossil and, in order to do so, scientists use absolute dating methods.
Most absolute dates are obtained through radiometric dating methods, which is based on radioactive isotopes that decay over time. Isotopes are elements that have the same number of protons but a different number of neutrons. When an isotope is not stable, it will decay at a fixed rate, which is different for each isotope. One of the most common isotopic dating methods used is radiocarbon dating, which relies on the decay of C-14 (a radioactive isotope of carbon) to N-14 (stable isotope of nitrogen). With this method, scientists can only date organic material that contains carbon like bone or charcoal. Also, because of the time it takes for C-14 to decay, this dating method can only date material that are younger than about 40,000 years old!
Another common radiometric dating method used is K-Ar (Potassium-Argon) or 40Ar-39Ar (Argon-Argon), which dates minerals (e.g., feldspar) found in volcanic material deposited during the time the fossil was living. A great example of this can be seen with our dear friend “Lucy.” Geologists were able to extract feldspar minerals from a layer of volcanic ash right below the stratigraphic layer that Lucy was found in and used them to date the deposit. Using 40Ar/39Ar as the radioactive clock, the deposits were dated to roughly 3.18 million years old. Without that volcanic layer and radiometric methods, we would have never known how old Lucy really was!

The stratifications or layers in the hillside at Hadar, Ethiopia, show the volcanic material to date the fossil skeleton, “Lucy.”
Image credit Donald Johanson
When was the last time you saw the light?
While radiocarbon (C-14) and Ar-Ar dating are great techniques, sometimes material with radioactive isotopes are not readily available to use. When this happens, geologists will often turn to dating methods like thermoluminescence or optically stimulated luminescence, which rely on electrons becoming “trapped” in the crystal structure of specific minerals (e.g., quartz). When the minerals are stimulated by light or heat, they will release the electrons in the form of light (i.e., luminescence). The amount of energy that is released, as well as the rate at which the energy accumulated, is used to determine how long the material was buried from sunlight (i.e., optically stimulated) or last heated (i.e., thermo-stimulated). Like most methods, these luminescence techniques have a limit use and are only capable of dating sediments that are a few hundred years old to hundreds of thousands of years old.
Try a few ways
While there are various dating methods that geologists can use, it is always important to use as many dating techniques as possible to make sure the correct age is calculated and to help build a greater context for that fossil. Without these dating methods, we would never fully understand when Lucy once lived, the timing of certain climatic shifts, and essentially our entire evolutionary past. With the help of relative and absolute dating methods, understanding the timing of these events is possible!
Written by Jayde Hirniak